دورة المؤشرنت للتحليل الفني
50 دينار كويتي
أنت تستخدم أحد المتصفحات القديمة. قد لا يتم عرض هذا الموقع أو المواقع الأخرى بشكل صحيح.
يجب عليك ترقية متصفحك أو استخدام أحد المتصفحات البديلة.
يجب عليك ترقية متصفحك أو استخدام أحد المتصفحات البديلة.
تابع معي يوميا
- بادئ الموضوع canada
- تاريخ البدء
canada
عضو نشط
السلام عليكم
في خصوص palm بعد ما شرتها hp شنو التوقعات للسهم والمساهمين
خبرتنا قليله في هذا الموضوع
وشكرا
لاتبيع
Top 10 Fastest-Growing Telecom Equipment Stocks: ARUN, CMTL, EXFO, PALM, OPLK, RVBD, ALVR, BDC, XXIA, IDCC (May 09, 2010)
http://www.cnanalyst.com/2010/05/to...o-palm-oplk-rvbd-alvr-bdc-xxia-idcc-may-.html
Palm (NASDAQ
 ALM) traded in a range yesterday that spanned from a low of $5.63 to a high of $5.76. Yesterday's low of the day pierced the 3-day low of $5.70 on volume of 21.9 million shares.
ALM) traded in a range yesterday that spanned from a low of $5.63 to a high of $5.76. Yesterday's low of the day pierced the 3-day low of $5.70 on volume of 21.9 million shares.http://www.mysmartrend.com/news-briefs/news-watch/palm-falls-thru-570-enters-new-trading-range-palm
canada
عضو نشط
هذا الخبر يوم 29/3 ارجو ان لاتسال كثير
عندك النت وابحث عن الاخبار
انت مستثمر حافظ على فلوسك بجمع الاخبار حولها
وتحليلها اذا كانت خبرتك قليله لاتضيع فلوسك على حسب التوصيات
توصياتي للمضاربه اليوميه والسريعه
شكرا
A huge tech merger was announced last night after the bell…HP (NYSE:HPQ) said it has agreed to buy struggling smartphone maker Palm (NASDAQ ALM) for $1.2 billion.
ALM) for $1.2 billion.
The cash deal values Palm at $5.70 per share, more than a 20% premium over its closing price Thursday.
HP said the combination of its global scale and financial strength with Palm’s webOS platform will boost HP’s ability to compete more aggressively in the large and profitable smartphone arena.
Palm has struggled in recent months to compete with Apple’s (AAPL) and RIM’s (RIMM) more popular smartphone options.
The boards of both companies have already approved the deal, but is subject to approval by Palm shareholder and regulators. The companies expect the deal to close during HP’s third fiscal quarter, which ends July 31.
عندك النت وابحث عن الاخبار
انت مستثمر حافظ على فلوسك بجمع الاخبار حولها
وتحليلها اذا كانت خبرتك قليله لاتضيع فلوسك على حسب التوصيات
توصياتي للمضاربه اليوميه والسريعه
شكرا
A huge tech merger was announced last night after the bell…HP (NYSE:HPQ) said it has agreed to buy struggling smartphone maker Palm (NASDAQ
 ALM) for $1.2 billion.
ALM) for $1.2 billion.The cash deal values Palm at $5.70 per share, more than a 20% premium over its closing price Thursday.
HP said the combination of its global scale and financial strength with Palm’s webOS platform will boost HP’s ability to compete more aggressively in the large and profitable smartphone arena.
Palm has struggled in recent months to compete with Apple’s (AAPL) and RIM’s (RIMM) more popular smartphone options.
The boards of both companies have already approved the deal, but is subject to approval by Palm shareholder and regulators. The companies expect the deal to close during HP’s third fiscal quarter, which ends July 31.
KuwaitEmail
عضو نشط
- التسجيل
- 24 مايو 2009
- المشاركات
- 279
مرحبا مولاييييييييي
عميييييي شلونك وشلونك المؤمنين طيبين ان شاء الله تعالى
عطني رايك بهذا الجنقل tchh
عليه كلام يحك الخد
اصبر عليه؟
ثانكيو,,
عسى ربي يسدد خطاياك ويمن عليك بالصحة والعافية اللهم آمين
عميييييي شلونك وشلونك المؤمنين طيبين ان شاء الله تعالى
عطني رايك بهذا الجنقل tchh
عليه كلام يحك الخد
اصبر عليه؟
ثانكيو,,
عسى ربي يسدد خطاياك ويمن عليك بالصحة والعافية اللهم آمين
canada
عضو نشط
معذره اخي الطيب
ان ماعندي خبره في تحليل البني ستوك
ولاا اتعامل بها
لاناها خدعه ومضيعه فلوس
اغلبها شركات وهميه لجمع المال السريع
انا اكون سعيد في خدمتك في غيرها
عده اشخاص سالوني عنها هذا الصباح
ولم اجيب عنها
انا اسف لعدم الاستطاعه على الرد في الجواب
ان ماعندي خبره في تحليل البني ستوك
ولاا اتعامل بها
لاناها خدعه ومضيعه فلوس
اغلبها شركات وهميه لجمع المال السريع
انا اكون سعيد في خدمتك في غيرها
عده اشخاص سالوني عنها هذا الصباح
ولم اجيب عنها
انا اسف لعدم الاستطاعه على الرد في الجواب
canada
عضو نشط
الاخ حبي الوحيد
تابع
pcln
تابع
pcln
canada
عضو نشط
حياك الله سيدي الكريم بو علي
canada
عضو نشط
تابع معي والرزق من الله
يوم 11\5
jaso
trgl
agen
cgen
opxa
fosl
dsco
crus
يوم 11\5
jaso
trgl
agen
cgen
opxa
fosl
dsco
crus
canada
عضو نشط
بالتوفيق والرزق اخي الكريم
انا في الخدمه
مضاربه فقط
انا في الخدمه
مضاربه فقط
canada
عضو نشط
رجاء لاتسال الكثير
هذا الربورت
dsco
10-May-2010
Quarterly Report
Note 2 - Liquidity Risks and Management's Plans
We have incurred substantial losses since inception, due to investments in research and development, manufacturing and potential commercialization activities and we expect to continue to incur substantial losses over the next several years. Historically, we have funded our business operations through various sources, including public and private securities offerings, draw downs under our Committed Equity Financing Facilities (CEFFs), capital equipment and debt facilities, and strategic alliances. We expect to continue to fund our business operations through a combination of these sources, as well as sales revenue from our product candidates, beginning with Surfaxin for the prevention of RDS, if approved.
Following receipt from the FDA of a Complete Response Letter for Surfaxin in April 2009, we made fundamental changes in our business strategy. We now believe that it is in our best interest financially to seek to develop and commercialize our KL4 technology through strategic alliances or other collaboration arrangements, including in the United States. However, there can be no assurance that any strategic alliance or other arrangement will be successfully concluded.
The accompanying interim unaudited consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As a result of our cash position as of December 31, 2009, the audit opinion we received from our independent auditors for the year ended December 31, 2009 contains a notation related to our ability to continue as a going concern. Our ability to continue as a going concern is dependent on our ability to raise additional capital, to fund our research and development and commercial programs and meet our obligations on a timely basis. If we are unable to successfully raise sufficient additional capital, through strategic and collaborative arrangements with potential partners and/or future debt and equity financings, we will likely not have sufficient cash flows and liquidity to fund our business operations, which could significantly limit our ability to continue as a going concern. In that event, we may be forced to further limit development of many, if not all, of our programs and consider other means of creating value for our stockholders, such as licensing the development and/or commercialization of products that we consider valuable and might otherwise plan to develop ourselves. If we are unable to raise the necessary capital, we may be forced to curtail all of our activities and, ultimately, cease operations. Even if we are able to raise additional capital, such financings may only be available on unattractive terms, or could result in significant dilution of stockholders' interests and, in such event, the market price of our common stock may decline. Our financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue in existence.
Our future capital requirements will depend upon many factors, including our efforts to secure one or more strategic alliances to support our product development activities and commercialization plans, and the ultimate success of our product development and commercialization plans. Currently, we are focused on developing our lead KL4 surfactant products to address the most significant respiratory conditions affecting pediatric populations. However, there can be no assurance that our research and development projects will be successful, that products developed will obtain necessary regulatory approval, that any approved product will be commercially viable, that any CEFF will be available for future financings, or that we will be able to secure strategic alliances or obtain additional capital when needed on acceptable terms, if at all. Even if we succeed in securing strategic alliances, raising additional capital and developing and subsequently commercializing product candidates, we may never achieve sufficient sales revenue to achieve or maintain profitability.
As of March 31, 2010, we had cash and cash equivalents of $24.2 million, which includes net proceeds of $15.1 million ($16.5 million gross) from a public offering that we completed in February 2010. As of May 7, 2010, neither the May 2008 CEFF nor the December 2008 CEFF was available to us because the closing market price of our common stock ($0.47) was below the minimum price required ($1.15 and $0.60, respectively) to utilize the facility. If and when the CEFFs become available, we may potentially raise (subject to certain conditions, including minimum stock price and volume limitations) up to an aggregate of $69.5 million. See, Note 4 - Stockholders' Equity, for details about our CEFFs.
As of March 31, 2010, our $10.5 million loan with PharmaBio Development Inc (PharmaBio), the former strategic investment subsidiary of Quintiles Transnational Corp. (Quintiles), was classified as a current liability payable on April 30, 2010. On April 28, 2010, we completed a restructuring of the loan ($10.6M at the time of restructuring) pursuant to a Payment Agreement and Loan Amendment dated April 27, 2010 (PharmaBio Agreement) that provided for
(a) payment in cash of an aggregate of $6.6 million, representing $4.5 million in outstanding principal and $2.1 million in accrued interest, (b) a maturity date extension for the remaining $4 million principal amount under the loan, $2 million of which now will be due and payable on July 30, 2010 and the remaining $2 million of which will be due and payable on September 30, 2010, and
(c) so long as we timely make each of the remaining principal payments on or before their respective due dates, no further interest will accrue on the outstanding principal amount. In addition, we agreed to maintain (i) at least $10 million in cash and cash equivalents until payment of the first $2 million installment is made on or before July 30, 2010, and (ii) at least $8 million in cash and cash equivalents until the payment of the second $2 million installment on or before September 30, 2010, after which the PharmaBio loan will be paid in full. Also under the PharmaBio Agreement, PharmaBio surrendered to us for cancellation warrants to purchase an aggregate of 2,393,612 shares of our common stock that we had issued previously to PharmaBio in connection with the PharmaBio loan and a previous offering of securities. See, Note 8 - Subsequent Events.
The PharmaBio Agreement also provides that we and PharmaBio will negotiate in good faith to potentially enter into a strategic arrangement under which PharmaBio would provide funding for a research collaboration between Quintiles and us relating to the possible research and development, and commercialization of two of our drug product candidates, Surfaxin LS and Aerosurf, for the prevention and treatment of RDS in premature infants. However, neither party is obligated to enter into any such arrangement except to the extent that the parties, in their individual and sole discretion, enter into definitive documents with respect thereto. Accordingly, there can be no assurances that any such arrangement will be completed. See, Note 8 - Subsequent Events.
Also on April 27, 2010, we entered into a Securities Purchase Agreement pursuant to which PharmaBio agreed to purchase 4,052,312 shares of our common stock and warrants to purchase an aggregate of 2,026,156 shares of common stock, resulting in gross proceeds to us, on April 29, 2010, of $2.2 million ($2.1 million net). The shares of common stock and warrants were sold as units, with each unit consisting of (a) one share of common stock, and (b) one-half of a warrant to purchase a share of common stock, at an offering price of $0.5429 per unit. The warrants generally will be exercisable beginning 181 days after the date of issuance for a period of five years from the original date of issuance at an exercise price of $0.7058 per share. See, Note 4 - Stockholders' Equity, and Note 8 - Subsequent Events.
Note 3 - Accounting Policies and Recent Accounting Pronouncements
Accounting policies
There have been no changes to our critical accounting policies since December 31, 2009. For more information on critical accounting policies, see, Note 3 - "Summary of Significant Accounting Policies and Recent Accounting Pronouncements" to the consolidated financial statements included in our 2009 Annual Report on Form 10-K. Readers are encouraged to review those disclosures in conjunction with the review of this Form 10-Q.
Net loss per common share
Basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the periods. As of March 31, 2010 and 2009, 44.0 million and 24.8 million shares of common stock, respectively, were potentially issuable upon the exercise of certain stock options and warrants. Due to our net loss, these potentially issuable shares were not included in the calculation of diluted net loss per share as the effect would be anti-dilutive, therefore basic and dilutive net loss per share are the same.
Comprehensive loss
Comprehensive loss consists of net loss plus the changes in unrealized gains and losses on available-for-sale securities. Comprehensive loss for the three months ended March 31, 2010 and 2009 are as follows:
For the three months ended
(in thousands) March 31,
2010 2009
Net loss $ (7,288 ) $ (9,000 )
Change in unrealized gains / (losses) on marketable securities - (1 )
Comprehensive loss $ (7,288 ) $ (9,001 )
Recent accounting pronouncements
In March 2010, ASU 2010-17, Revenue Recognition-Milestone Method (Topic 605):
Milestone Method of Revenue Recognition-a consensus of the FASB Emerging Issues Task Force ("ASU 2010-17") was issued and will amend the accounting for revenue arrangements under which a vendor satisfies its performance obligations to a customer over a period of time, when the deliverable or unit of accounting is not within the scope of other authoritative literature, and when the arrangement consideration is contingent upon the achievement of a milestone. The amendment defines a milestone and clarifies whether an entity may recognize consideration earned from the achievement of a milestone in the period in which the milestone is achieved. This amendment is effective for fiscal years beginning on or after June 15, 2010, with early adoption permitted. The amendment may be applied retrospectively to all arrangements or prospectively for milestones achieved after the effective date. We do not believe the adoption of this ASU will have a material impact on our financial statements.
Note 4 - Stockholders' Equity
Registered Public Offerings
In February 2010, we completed a public offering of 27.5 million shares of our common stock and warrants to purchase 13.8 million shares of our common stock, sold as units, with each unit consisting of one share of common stock and a warrant to purchase 0.5 of a share of common stock, at a public offering price of $0.60 per unit, resulting in gross proceeds to us of $16.5 million ($15.1 million net). This offering was made pursuant to a prospectus supplement dated April 28, 2010 and an accompanying prospectus dated June 18, 2008 pursuant to our existing shelf registration statement on Form S-3 (File No. 333-151654), which was filed with the SEC on June 13, 2008 and declared effective by the SEC on June 18, 2008 (2008 Shelf Registration Statement). The warrants expire in February 2015 and are exercisable, subject to an aggregate beneficial ownership limitation, at a price per share of $0.85. The exercise price and number of shares of common stock issuable on exercise of the warrants will be subject to adjustment in the event of any stock split, reverse stock split, stock dividend, recapitalization, reorganization or similar transaction. The exercise price and the amount and/or type of property to be issued upon exercise of the warrants will also be subject to adjustment if the Company engages in a "Fundamental Transaction" (as defined in the form of warrant). The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis.
In May 2009, we completed a registered direct public offering of 14.0 million shares of our common stock and warrants to purchase seven million shares of common stock, sold as units to select institutional investors, with each unit consisting of one share and a warrant to purchase 0.5 of a share of common stock, at a price of $0.81 per unit, resulting in gross proceeds to us of $11.3 million ($10.5 million net). This offering was made pursuant to a prospectus supplement dated May 8, 2009 to the prospectus dated June 18, 2008 included in our 2008 Shelf Registration Statement. The warrants expire in May 2014 and are exercisable at a price per share of $1.15. The exercise price and number of shares of common stock issuable on exercise of the warrants will be subject to adjustment in the event of any stock split, reverse stock split, stock dividend, recapitalization, reorganization or similar transaction. The exercise price and the amount and/or type of property to be issued upon exercise of the warrants will also be subject to adjustment if the Company engages in a "Fundamental Transaction" (as defined in the form of warrant). The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis.
Common Stock Offering with PharmaBio Development Inc.
On April 27, 2010, we entered into a Securities Purchase Agreement with PharmaBio, as the sole purchaser, related to an offering of 4,052,312 shares of common stock and warrants to purchase an aggregate of 2,026,156 shares of common stock, sold as units, with each unit consisting of one share of common stock and one half of a warrant to purchase a share of common stock, at an offering price of $0.5429 per unit, representing the greater of (a) the volume-weighted average sale price ("VWAP") per share of the common stock on The Nasdaq Global Market for the 20 trading days ending on April 27, 2010 and (b) the last reported closing price of $0.5205 per share of the common stock on The Nasdaq Global Market on such date. The offering resulted in gross proceeds to us of $2.2 million ($2.1 million net). This offering was made pursuant to a prospectus supplement dated April 28, 2010 to the prospectus dated June 18, 2008 included in our 2008 Shelf Registration Statement. The warrants expire in April 2015 and generally will be exercisable beginning 181 days after the date of issuance, subject to an aggregate beneficial ownership limitation of 9.9%, at a price per share of $0.7058, which represents a 30% premium to the VWAP for the 20 trading days ending on April 27, 2010. The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis. See also, Note 8 - Subsequent Events.
Committed Equity Financing Facilities(CEFFs)
As of March 31, 2010, we had two CEFFs with Kingsbridge Capital Limited (Kingsbridge), under which Kingsbridge is committed to purchase, subject to certain conditions, newly-issued shares of our common stock. The CEFFs, dated December 12, 2008 (December 2008 CEFF) and May 22, 2008 (May 2008 CEFF), allow us at our discretion to raise capital for a period of three years ending February 6, 2011 and June 18, 2011, respectively, at the time and in amounts deemed suitable to us. We are not obligated to utilize any of the funds available under the CEFFs. Our ability to access funds available under the CEFFs is subject to certain conditions, including stock price and volume limitations.
Under the December 2008 CEFF, as of March 31, 2010, we had 7.1 million shares potentially available for issuance (up to a maximum of $17.7 million), provided that the VWAP of our common stock on each trading day must be at least equal to the greater of (i) $.60 or (ii) 90% of the closing price of our common stock on the trading day immediately preceding the draw down period (Minimum VWAP). Under the May 2008 CEFF, as of March 31, 2010, we had approximately 12.8 million shares potentially available for issuance (up to a maximum of $51.7 million), provided that the VWAP on each trading day must be at least equal to the greater of $1.15 or the Minimum VWAP. Use of each CEFF is subject to certain other covenants and conditions, including aggregate share and dollar limitations for each draw down. See, "Item 7 -Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources - Committed Equity Financing Facilities (CEFFs)" included in our 2009 Annual Report on Form 10-K. As of May 7, 2010, neither CEFF is currently available because the market price of our common stock is less than the minimum price required to utilize either CEFF.
To date, we have not utilized our CEFFs in 2010. During 2009, we raised an aggregate of $10.7 million from 10 draw-downs under our CEFFs. If and when the closing market price of our common stock is at least equal to the minimum price required under our CEFFs, we anticipate using them to support our working capital needs and maintain cash availability in 2010.
Note 5 - Fair Value of Financial Instruments
We adopted the provisions of ASC Topic 820, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value under GAAP and enhances disclosures about fair value measurements.
Under ASC Topic 820, fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy is based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
� Level 1 - Quoted prices in active markets for identical assets and liabilities. Level 1 is generally considered the most reliable measurement of fair value under ASC 820.
� Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
� Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Fair Value on a Recurring Basis
Due to their short-term maturity, the carrying amounts of cash, money markets
and accounts payable approximate their fair values. The table below categorized
assets measured at fair value on a recurring basis based upon the lowest level
of significant input (Level 1) to the valuations as of March 31, 2010
Fair Value Fair value measurement using
March 31,
Assets 2010 Level 1 Level 2 Level 3
Money Markets and Certificates of Deposit $ 21,890 $ 21,890 $ - $ -
Restricted Cash 400 400 - -
Total $ 22,290 $ 22,290 $ - $ -
Note 6 - Stock Options and Stock-Based Employee Compensation
We recognize all share-based payments to employees and non-employee directors in our financial statements based on their grant date fair values, calculated using the Black-Scholes option pricing model. Compensation expense related to share-based awards is recognized ratably over the requisite service period, typically three years for employees.
The fair value of each option award is estimated on the date of grant using the Black-Scholes option-pricing formula that uses weighted average assumptions noted in the following table.
March 31, March 31,
2010 2009
Expected volatility 99% 81%
Expected term 4.7 years 4.6 years
Risk-free interest rate 1.7% 2.1%
Expected dividends - -
The total employee stock-based compensation for the three months ended March 31, 2010 and 2009 was as follows:
Three Months Ended
March 31,
(in thousands) 2010 2009
Research & Development $ 166 $ 209
General & Administrative 232 670
Total $ 398 $ 879
As of March 31, 2010, there was $1.8 million of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Amended and Restated 1998 Stock Incentive Plan (1998 Plan) and the 2007 Long-Term Incentive Plan (2007 Plan). That cost is expected to be recognized over a weighted-average vesting period of 1.1 years.
Note 7 - Contractual Obligations and Commitments
Former CEO Commitment
In connection with the resignation in August 2009 of Robert J. Capetola, Ph.D., our former President, Chief Executive Officer and member of our Board of Directors, we entered into a separation agreement and general release (the "Separation Agreement") dated August 13, 2009, that provided, among other things, for periodic severance payments through the earlier of (i) May 3, 2010 (Severance Period) or (ii) the date, if ever, of a Corporate Transaction (defined below). Under the Separation Agreement, if a Corporate Transaction were to occur during the Severance Period, Dr. Capetola would become entitled to receive an additional severance payment of up to $1,580,000 or, if any such Corporate Transaction were to constitute a Change of Control, a payment of up to $1,777,500; provided, however, that in each case, any such payment is reduced by the sum of the aggregate cash severance amounts already paid under the Separation Agreement.
A "Corporate Transaction" was defined in the Separation Agreement to include one or more public or private financings that were completed during the Severance Period and resulted in cash proceeds (net of transaction costs) to us of at least $20 million received during the Severance Period or within 90 calendar days thereafter. From August 13, 2009 through February 23, 2010, we raised approximately $21.0 million of aggregate net proceeds, consisting of approximately $5.9 million from financing transactions under our CEFFs throughout the period and $15.1 million from a public offering that was completed on February 23, 2010. As these transactions satisfied the criteria for a Corporate Transaction under the Separation Agreement, on March 3, 2010, we paid to Dr. Capetola an additional $1.06 million (less withholding), representing $1.58 million reduced by the sum of the cash severance amounts previously paid under the Separation Agreement, which totaled approximately $0.52 million. At this time, our obligation to make periodic payments under the Separation Agreement has been satisfied and no further payments are due to Dr. Capetola.
The full text of the Separation Agreement is attached to our Current Report on Form 8-K that we filed with the SEC on August 19, 2009. For a summary of the Separation Agreement, see, "Item 11- Executive Compensation -Resignation of our President and Chief Executive Officer," in our Amendment No. 1 to our 2009 Annual Report on Form 10-K that we filed with the SEC on April 30, 2010 (2009 Form 10-K/A).
Note 8- Subsequent Events
We evaluated all events or transactions that occurred after March 31, 2010 up through the date we issued these financial statements. During this period we did not have any material recognized subsequent events, however, there was one nonrecognized subsequent event described below:
Loan Restructuring - PharmaBio Development Inc.
As of March 31, 2010, our $10.5 million loan with PharmaBio was classified as a current liability, payable on April 30, 2010. On April 27, 2010, we entered into the PharmaBio Agreement and on April 28, 2010, completed a restructuring of the loan ($10.6M at the time of restructuring). The PharmaBio Agreement provided for
(a) payment in cash of an aggregate of $6.6 million, representing $4.5 million in outstanding principal and $2.1 million in accrued interest, (b) a maturity date extension for the remaining $4 million principal amount under the loan, $2 million of which now will be due and payable on July 30, 2010 and the remaining $2 million of which will be due and payable on September 30, 2010, and
(c) so long as we timely make each of the remaining principal payments on or before their respective due dates, no further interest will accrue on the outstanding principal amount. In addition, we agreed to maintain (i) at least $10 million in cash and cash equivalents until payment of the first $2 million installment is made on or before July 30, 2010, and (ii) at least $8 million in cash and cash equivalents until the payment of the second $2 million installment on or before September 30, 2010, after which the PharmaBio loan will be paid in full. Also under the PharmaBio Agreement, PharmaBio surrendered to us for cancellation the following warrants to purchase an aggregate of 2,393,612 shares of our common stock that we had issued previously to PharmaBio in connection with the PharmaBio loan and a previous offering of securities: a warrant to purchase 850,000 shares of common stock at $7.19 per share expiring on November 3, 2014, a warrant to purchase 1,500,000 shares of common stock at $3.58 per share expiring on October 26, 2013 and a warrant to purchase 43,612 shares of the Company's common stock at $6.875 per share expiring on September 19, 2010.
The PharmaBio Agreement also provided that we and PharmaBio would negotiate in good faith to potentially enter into a strategic arrangement under which PharmaBio would provide funding for a research collaboration between Quintiles and us relating to the possible research and development, and commercialization of two of our drug product candidates, Surfaxin LS and Aerosurf, for the prevention and treatment of RDS in premature infants. However, neither party is obligated to enter into any such arrangement except to the extent that the . . .
هذا الربورت
dsco
10-May-2010
Quarterly Report
Note 2 - Liquidity Risks and Management's Plans
We have incurred substantial losses since inception, due to investments in research and development, manufacturing and potential commercialization activities and we expect to continue to incur substantial losses over the next several years. Historically, we have funded our business operations through various sources, including public and private securities offerings, draw downs under our Committed Equity Financing Facilities (CEFFs), capital equipment and debt facilities, and strategic alliances. We expect to continue to fund our business operations through a combination of these sources, as well as sales revenue from our product candidates, beginning with Surfaxin for the prevention of RDS, if approved.
Following receipt from the FDA of a Complete Response Letter for Surfaxin in April 2009, we made fundamental changes in our business strategy. We now believe that it is in our best interest financially to seek to develop and commercialize our KL4 technology through strategic alliances or other collaboration arrangements, including in the United States. However, there can be no assurance that any strategic alliance or other arrangement will be successfully concluded.
The accompanying interim unaudited consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As a result of our cash position as of December 31, 2009, the audit opinion we received from our independent auditors for the year ended December 31, 2009 contains a notation related to our ability to continue as a going concern. Our ability to continue as a going concern is dependent on our ability to raise additional capital, to fund our research and development and commercial programs and meet our obligations on a timely basis. If we are unable to successfully raise sufficient additional capital, through strategic and collaborative arrangements with potential partners and/or future debt and equity financings, we will likely not have sufficient cash flows and liquidity to fund our business operations, which could significantly limit our ability to continue as a going concern. In that event, we may be forced to further limit development of many, if not all, of our programs and consider other means of creating value for our stockholders, such as licensing the development and/or commercialization of products that we consider valuable and might otherwise plan to develop ourselves. If we are unable to raise the necessary capital, we may be forced to curtail all of our activities and, ultimately, cease operations. Even if we are able to raise additional capital, such financings may only be available on unattractive terms, or could result in significant dilution of stockholders' interests and, in such event, the market price of our common stock may decline. Our financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue in existence.
Our future capital requirements will depend upon many factors, including our efforts to secure one or more strategic alliances to support our product development activities and commercialization plans, and the ultimate success of our product development and commercialization plans. Currently, we are focused on developing our lead KL4 surfactant products to address the most significant respiratory conditions affecting pediatric populations. However, there can be no assurance that our research and development projects will be successful, that products developed will obtain necessary regulatory approval, that any approved product will be commercially viable, that any CEFF will be available for future financings, or that we will be able to secure strategic alliances or obtain additional capital when needed on acceptable terms, if at all. Even if we succeed in securing strategic alliances, raising additional capital and developing and subsequently commercializing product candidates, we may never achieve sufficient sales revenue to achieve or maintain profitability.
As of March 31, 2010, we had cash and cash equivalents of $24.2 million, which includes net proceeds of $15.1 million ($16.5 million gross) from a public offering that we completed in February 2010. As of May 7, 2010, neither the May 2008 CEFF nor the December 2008 CEFF was available to us because the closing market price of our common stock ($0.47) was below the minimum price required ($1.15 and $0.60, respectively) to utilize the facility. If and when the CEFFs become available, we may potentially raise (subject to certain conditions, including minimum stock price and volume limitations) up to an aggregate of $69.5 million. See, Note 4 - Stockholders' Equity, for details about our CEFFs.
As of March 31, 2010, our $10.5 million loan with PharmaBio Development Inc (PharmaBio), the former strategic investment subsidiary of Quintiles Transnational Corp. (Quintiles), was classified as a current liability payable on April 30, 2010. On April 28, 2010, we completed a restructuring of the loan ($10.6M at the time of restructuring) pursuant to a Payment Agreement and Loan Amendment dated April 27, 2010 (PharmaBio Agreement) that provided for
(a) payment in cash of an aggregate of $6.6 million, representing $4.5 million in outstanding principal and $2.1 million in accrued interest, (b) a maturity date extension for the remaining $4 million principal amount under the loan, $2 million of which now will be due and payable on July 30, 2010 and the remaining $2 million of which will be due and payable on September 30, 2010, and
(c) so long as we timely make each of the remaining principal payments on or before their respective due dates, no further interest will accrue on the outstanding principal amount. In addition, we agreed to maintain (i) at least $10 million in cash and cash equivalents until payment of the first $2 million installment is made on or before July 30, 2010, and (ii) at least $8 million in cash and cash equivalents until the payment of the second $2 million installment on or before September 30, 2010, after which the PharmaBio loan will be paid in full. Also under the PharmaBio Agreement, PharmaBio surrendered to us for cancellation warrants to purchase an aggregate of 2,393,612 shares of our common stock that we had issued previously to PharmaBio in connection with the PharmaBio loan and a previous offering of securities. See, Note 8 - Subsequent Events.
The PharmaBio Agreement also provides that we and PharmaBio will negotiate in good faith to potentially enter into a strategic arrangement under which PharmaBio would provide funding for a research collaboration between Quintiles and us relating to the possible research and development, and commercialization of two of our drug product candidates, Surfaxin LS and Aerosurf, for the prevention and treatment of RDS in premature infants. However, neither party is obligated to enter into any such arrangement except to the extent that the parties, in their individual and sole discretion, enter into definitive documents with respect thereto. Accordingly, there can be no assurances that any such arrangement will be completed. See, Note 8 - Subsequent Events.
Also on April 27, 2010, we entered into a Securities Purchase Agreement pursuant to which PharmaBio agreed to purchase 4,052,312 shares of our common stock and warrants to purchase an aggregate of 2,026,156 shares of common stock, resulting in gross proceeds to us, on April 29, 2010, of $2.2 million ($2.1 million net). The shares of common stock and warrants were sold as units, with each unit consisting of (a) one share of common stock, and (b) one-half of a warrant to purchase a share of common stock, at an offering price of $0.5429 per unit. The warrants generally will be exercisable beginning 181 days after the date of issuance for a period of five years from the original date of issuance at an exercise price of $0.7058 per share. See, Note 4 - Stockholders' Equity, and Note 8 - Subsequent Events.
Note 3 - Accounting Policies and Recent Accounting Pronouncements
Accounting policies
There have been no changes to our critical accounting policies since December 31, 2009. For more information on critical accounting policies, see, Note 3 - "Summary of Significant Accounting Policies and Recent Accounting Pronouncements" to the consolidated financial statements included in our 2009 Annual Report on Form 10-K. Readers are encouraged to review those disclosures in conjunction with the review of this Form 10-Q.
Net loss per common share
Basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding for the periods. As of March 31, 2010 and 2009, 44.0 million and 24.8 million shares of common stock, respectively, were potentially issuable upon the exercise of certain stock options and warrants. Due to our net loss, these potentially issuable shares were not included in the calculation of diluted net loss per share as the effect would be anti-dilutive, therefore basic and dilutive net loss per share are the same.
Comprehensive loss
Comprehensive loss consists of net loss plus the changes in unrealized gains and losses on available-for-sale securities. Comprehensive loss for the three months ended March 31, 2010 and 2009 are as follows:
For the three months ended
(in thousands) March 31,
2010 2009
Net loss $ (7,288 ) $ (9,000 )
Change in unrealized gains / (losses) on marketable securities - (1 )
Comprehensive loss $ (7,288 ) $ (9,001 )
Recent accounting pronouncements
In March 2010, ASU 2010-17, Revenue Recognition-Milestone Method (Topic 605):
Milestone Method of Revenue Recognition-a consensus of the FASB Emerging Issues Task Force ("ASU 2010-17") was issued and will amend the accounting for revenue arrangements under which a vendor satisfies its performance obligations to a customer over a period of time, when the deliverable or unit of accounting is not within the scope of other authoritative literature, and when the arrangement consideration is contingent upon the achievement of a milestone. The amendment defines a milestone and clarifies whether an entity may recognize consideration earned from the achievement of a milestone in the period in which the milestone is achieved. This amendment is effective for fiscal years beginning on or after June 15, 2010, with early adoption permitted. The amendment may be applied retrospectively to all arrangements or prospectively for milestones achieved after the effective date. We do not believe the adoption of this ASU will have a material impact on our financial statements.
Note 4 - Stockholders' Equity
Registered Public Offerings
In February 2010, we completed a public offering of 27.5 million shares of our common stock and warrants to purchase 13.8 million shares of our common stock, sold as units, with each unit consisting of one share of common stock and a warrant to purchase 0.5 of a share of common stock, at a public offering price of $0.60 per unit, resulting in gross proceeds to us of $16.5 million ($15.1 million net). This offering was made pursuant to a prospectus supplement dated April 28, 2010 and an accompanying prospectus dated June 18, 2008 pursuant to our existing shelf registration statement on Form S-3 (File No. 333-151654), which was filed with the SEC on June 13, 2008 and declared effective by the SEC on June 18, 2008 (2008 Shelf Registration Statement). The warrants expire in February 2015 and are exercisable, subject to an aggregate beneficial ownership limitation, at a price per share of $0.85. The exercise price and number of shares of common stock issuable on exercise of the warrants will be subject to adjustment in the event of any stock split, reverse stock split, stock dividend, recapitalization, reorganization or similar transaction. The exercise price and the amount and/or type of property to be issued upon exercise of the warrants will also be subject to adjustment if the Company engages in a "Fundamental Transaction" (as defined in the form of warrant). The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis.
In May 2009, we completed a registered direct public offering of 14.0 million shares of our common stock and warrants to purchase seven million shares of common stock, sold as units to select institutional investors, with each unit consisting of one share and a warrant to purchase 0.5 of a share of common stock, at a price of $0.81 per unit, resulting in gross proceeds to us of $11.3 million ($10.5 million net). This offering was made pursuant to a prospectus supplement dated May 8, 2009 to the prospectus dated June 18, 2008 included in our 2008 Shelf Registration Statement. The warrants expire in May 2014 and are exercisable at a price per share of $1.15. The exercise price and number of shares of common stock issuable on exercise of the warrants will be subject to adjustment in the event of any stock split, reverse stock split, stock dividend, recapitalization, reorganization or similar transaction. The exercise price and the amount and/or type of property to be issued upon exercise of the warrants will also be subject to adjustment if the Company engages in a "Fundamental Transaction" (as defined in the form of warrant). The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis.
Common Stock Offering with PharmaBio Development Inc.
On April 27, 2010, we entered into a Securities Purchase Agreement with PharmaBio, as the sole purchaser, related to an offering of 4,052,312 shares of common stock and warrants to purchase an aggregate of 2,026,156 shares of common stock, sold as units, with each unit consisting of one share of common stock and one half of a warrant to purchase a share of common stock, at an offering price of $0.5429 per unit, representing the greater of (a) the volume-weighted average sale price ("VWAP") per share of the common stock on The Nasdaq Global Market for the 20 trading days ending on April 27, 2010 and (b) the last reported closing price of $0.5205 per share of the common stock on The Nasdaq Global Market on such date. The offering resulted in gross proceeds to us of $2.2 million ($2.1 million net). This offering was made pursuant to a prospectus supplement dated April 28, 2010 to the prospectus dated June 18, 2008 included in our 2008 Shelf Registration Statement. The warrants expire in April 2015 and generally will be exercisable beginning 181 days after the date of issuance, subject to an aggregate beneficial ownership limitation of 9.9%, at a price per share of $0.7058, which represents a 30% premium to the VWAP for the 20 trading days ending on April 27, 2010. The warrants are exercisable for cash only, except that if the related registration statement or an exemption from registration is not available for the resale of the warrant shares, the holder may exercise on a cashless basis. See also, Note 8 - Subsequent Events.
Committed Equity Financing Facilities(CEFFs)
As of March 31, 2010, we had two CEFFs with Kingsbridge Capital Limited (Kingsbridge), under which Kingsbridge is committed to purchase, subject to certain conditions, newly-issued shares of our common stock. The CEFFs, dated December 12, 2008 (December 2008 CEFF) and May 22, 2008 (May 2008 CEFF), allow us at our discretion to raise capital for a period of three years ending February 6, 2011 and June 18, 2011, respectively, at the time and in amounts deemed suitable to us. We are not obligated to utilize any of the funds available under the CEFFs. Our ability to access funds available under the CEFFs is subject to certain conditions, including stock price and volume limitations.
Under the December 2008 CEFF, as of March 31, 2010, we had 7.1 million shares potentially available for issuance (up to a maximum of $17.7 million), provided that the VWAP of our common stock on each trading day must be at least equal to the greater of (i) $.60 or (ii) 90% of the closing price of our common stock on the trading day immediately preceding the draw down period (Minimum VWAP). Under the May 2008 CEFF, as of March 31, 2010, we had approximately 12.8 million shares potentially available for issuance (up to a maximum of $51.7 million), provided that the VWAP on each trading day must be at least equal to the greater of $1.15 or the Minimum VWAP. Use of each CEFF is subject to certain other covenants and conditions, including aggregate share and dollar limitations for each draw down. See, "Item 7 -Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources - Committed Equity Financing Facilities (CEFFs)" included in our 2009 Annual Report on Form 10-K. As of May 7, 2010, neither CEFF is currently available because the market price of our common stock is less than the minimum price required to utilize either CEFF.
To date, we have not utilized our CEFFs in 2010. During 2009, we raised an aggregate of $10.7 million from 10 draw-downs under our CEFFs. If and when the closing market price of our common stock is at least equal to the minimum price required under our CEFFs, we anticipate using them to support our working capital needs and maintain cash availability in 2010.
Note 5 - Fair Value of Financial Instruments
We adopted the provisions of ASC Topic 820, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value under GAAP and enhances disclosures about fair value measurements.
Under ASC Topic 820, fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy is based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
� Level 1 - Quoted prices in active markets for identical assets and liabilities. Level 1 is generally considered the most reliable measurement of fair value under ASC 820.
� Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
� Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Fair Value on a Recurring Basis
Due to their short-term maturity, the carrying amounts of cash, money markets
and accounts payable approximate their fair values. The table below categorized
assets measured at fair value on a recurring basis based upon the lowest level
of significant input (Level 1) to the valuations as of March 31, 2010
Fair Value Fair value measurement using
March 31,
Assets 2010 Level 1 Level 2 Level 3
Money Markets and Certificates of Deposit $ 21,890 $ 21,890 $ - $ -
Restricted Cash 400 400 - -
Total $ 22,290 $ 22,290 $ - $ -
Note 6 - Stock Options and Stock-Based Employee Compensation
We recognize all share-based payments to employees and non-employee directors in our financial statements based on their grant date fair values, calculated using the Black-Scholes option pricing model. Compensation expense related to share-based awards is recognized ratably over the requisite service period, typically three years for employees.
The fair value of each option award is estimated on the date of grant using the Black-Scholes option-pricing formula that uses weighted average assumptions noted in the following table.
March 31, March 31,
2010 2009
Expected volatility 99% 81%
Expected term 4.7 years 4.6 years
Risk-free interest rate 1.7% 2.1%
Expected dividends - -
The total employee stock-based compensation for the three months ended March 31, 2010 and 2009 was as follows:
Three Months Ended
March 31,
(in thousands) 2010 2009
Research & Development $ 166 $ 209
General & Administrative 232 670
Total $ 398 $ 879
As of March 31, 2010, there was $1.8 million of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Amended and Restated 1998 Stock Incentive Plan (1998 Plan) and the 2007 Long-Term Incentive Plan (2007 Plan). That cost is expected to be recognized over a weighted-average vesting period of 1.1 years.
Note 7 - Contractual Obligations and Commitments
Former CEO Commitment
In connection with the resignation in August 2009 of Robert J. Capetola, Ph.D., our former President, Chief Executive Officer and member of our Board of Directors, we entered into a separation agreement and general release (the "Separation Agreement") dated August 13, 2009, that provided, among other things, for periodic severance payments through the earlier of (i) May 3, 2010 (Severance Period) or (ii) the date, if ever, of a Corporate Transaction (defined below). Under the Separation Agreement, if a Corporate Transaction were to occur during the Severance Period, Dr. Capetola would become entitled to receive an additional severance payment of up to $1,580,000 or, if any such Corporate Transaction were to constitute a Change of Control, a payment of up to $1,777,500; provided, however, that in each case, any such payment is reduced by the sum of the aggregate cash severance amounts already paid under the Separation Agreement.
A "Corporate Transaction" was defined in the Separation Agreement to include one or more public or private financings that were completed during the Severance Period and resulted in cash proceeds (net of transaction costs) to us of at least $20 million received during the Severance Period or within 90 calendar days thereafter. From August 13, 2009 through February 23, 2010, we raised approximately $21.0 million of aggregate net proceeds, consisting of approximately $5.9 million from financing transactions under our CEFFs throughout the period and $15.1 million from a public offering that was completed on February 23, 2010. As these transactions satisfied the criteria for a Corporate Transaction under the Separation Agreement, on March 3, 2010, we paid to Dr. Capetola an additional $1.06 million (less withholding), representing $1.58 million reduced by the sum of the cash severance amounts previously paid under the Separation Agreement, which totaled approximately $0.52 million. At this time, our obligation to make periodic payments under the Separation Agreement has been satisfied and no further payments are due to Dr. Capetola.
The full text of the Separation Agreement is attached to our Current Report on Form 8-K that we filed with the SEC on August 19, 2009. For a summary of the Separation Agreement, see, "Item 11- Executive Compensation -Resignation of our President and Chief Executive Officer," in our Amendment No. 1 to our 2009 Annual Report on Form 10-K that we filed with the SEC on April 30, 2010 (2009 Form 10-K/A).
Note 8- Subsequent Events
We evaluated all events or transactions that occurred after March 31, 2010 up through the date we issued these financial statements. During this period we did not have any material recognized subsequent events, however, there was one nonrecognized subsequent event described below:
Loan Restructuring - PharmaBio Development Inc.
As of March 31, 2010, our $10.5 million loan with PharmaBio was classified as a current liability, payable on April 30, 2010. On April 27, 2010, we entered into the PharmaBio Agreement and on April 28, 2010, completed a restructuring of the loan ($10.6M at the time of restructuring). The PharmaBio Agreement provided for
(a) payment in cash of an aggregate of $6.6 million, representing $4.5 million in outstanding principal and $2.1 million in accrued interest, (b) a maturity date extension for the remaining $4 million principal amount under the loan, $2 million of which now will be due and payable on July 30, 2010 and the remaining $2 million of which will be due and payable on September 30, 2010, and
(c) so long as we timely make each of the remaining principal payments on or before their respective due dates, no further interest will accrue on the outstanding principal amount. In addition, we agreed to maintain (i) at least $10 million in cash and cash equivalents until payment of the first $2 million installment is made on or before July 30, 2010, and (ii) at least $8 million in cash and cash equivalents until the payment of the second $2 million installment on or before September 30, 2010, after which the PharmaBio loan will be paid in full. Also under the PharmaBio Agreement, PharmaBio surrendered to us for cancellation the following warrants to purchase an aggregate of 2,393,612 shares of our common stock that we had issued previously to PharmaBio in connection with the PharmaBio loan and a previous offering of securities: a warrant to purchase 850,000 shares of common stock at $7.19 per share expiring on November 3, 2014, a warrant to purchase 1,500,000 shares of common stock at $3.58 per share expiring on October 26, 2013 and a warrant to purchase 43,612 shares of the Company's common stock at $6.875 per share expiring on September 19, 2010.
The PharmaBio Agreement also provided that we and PharmaBio would negotiate in good faith to potentially enter into a strategic arrangement under which PharmaBio would provide funding for a research collaboration between Quintiles and us relating to the possible research and development, and commercialization of two of our drug product candidates, Surfaxin LS and Aerosurf, for the prevention and treatment of RDS in premature infants. However, neither party is obligated to enter into any such arrangement except to the extent that the . . .
canada
عضو نشط
يابو تركي
تابع يوم غد
12\5
achn
http://finance.yahoo.com/news/Achillion-Announces-Positive-pz-83287319.html?x=0&.v=1
Achillion Pharmaceuticals, Inc. (Nasdaq:ACHN - News) today reported additional preliminary data from its Phase 1b clinical trial of ACH-1625, which demonstrated that both the third and fourth patient cohorts receiving treatment with ACH-1625 achieved meaningful reductions in HCV RNA after five-day monotherapy, with continued safety and tolerability in patients with hepatitis C (HCV). ACH-1625 is an inhibitor of HCV NS3 protease that was discovered and is being developed by Achillion.
smtx
http://finance.yahoo.com/news/SMTC-Reports-Positive-First-prnews-442627940.html?x=0&.v=4
nlst
http://www.reuters.com/article/idCNSGE64A0MB20100511?rpc=44
تابع يوم غد
12\5
achn
http://finance.yahoo.com/news/Achillion-Announces-Positive-pz-83287319.html?x=0&.v=1
Achillion Pharmaceuticals, Inc. (Nasdaq:ACHN - News) today reported additional preliminary data from its Phase 1b clinical trial of ACH-1625, which demonstrated that both the third and fourth patient cohorts receiving treatment with ACH-1625 achieved meaningful reductions in HCV RNA after five-day monotherapy, with continued safety and tolerability in patients with hepatitis C (HCV). ACH-1625 is an inhibitor of HCV NS3 protease that was discovered and is being developed by Achillion.
smtx
http://finance.yahoo.com/news/SMTC-Reports-Positive-First-prnews-442627940.html?x=0&.v=4
nlst
http://www.reuters.com/article/idCNSGE64A0MB20100511?rpc=44
canada
عضو نشط
الاخ Bull Rider الجواب على الخاص
canada
عضو نشط
تابع يوم غد
يوم 13/5
clne
aezs
aig
faz
dndn
rimm
bac
بالتوفيق والرزق
الاخ Bull Rider
انتبه
يوم 13/5
clne
aezs
aig
faz
dndn
rimm
bac
بالتوفيق والرزق
الاخ Bull Rider
انتبه
canada
عضو نشط
canada
عضو نشط
الاخ Bull Rider
TD
عندهم هل الخدمه
بس العموله اكثر
TD
عندهم هل الخدمه
بس العموله اكثر
canada
عضو نشط
ابو محمد
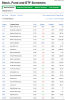
هذا فلتر للاسهم بالاسعار التى تريدها
اذا ما تعرف ضع بدايه السعر الذي تريده وفي الحقل التاني اعلى سعر ترغب فيه
ستخرج الاسهم التي طلبتها
ضع نكست واكمل الفراغات ستحصل على ماتريد
http://custom.marketwatch.com/custom/usatoday-com/screener/screener.asp?symb=
هذا فلتر للاسهم بالاسعار التى تريدها
اذا ما تعرف ضع بدايه السعر الذي تريده وفي الحقل التاني اعلى سعر ترغب فيه
ستخرج الاسهم التي طلبتها
ضع نكست واكمل الفراغات ستحصل على ماتريد
http://custom.marketwatch.com/custom/usatoday-com/screener/screener.asp?symb=
الملفات المرفقه:
canada
عضو نشط
لايوجد سهم اكثرمن 5 دولارز
الى لقاء يوم غد
بالتوفيق
الى لقاء يوم غد
بالتوفيق
canada
عضو نشط
تايع السهم التاليه ليوم 14\5
haup
ULBI
SSN
SOMX
PURE
siri
ZANE
AUTH
faz
etf
fas
rimm
dndn
abk
haup
ULBI
SSN
SOMX
PURE
siri
ZANE
AUTH
faz
etf
fas
rimm
dndn
abk

دورة المؤشرنت للتحليل الفني
50 دينار كويتي